Types of Adhesion
There are various mechanisms that can facilitate adhesion:- Chemical – this occurs when the surface atoms of two surfaces form a new compound that is intrinsically linked to both. This is how many liquid adhesive systems work such as cyanoacrylates or “SuperGlue”.
- Dispersive adhesion – this is a type of adhesion that relies on Van der Waals forces. Simply put, these weak forces occur in regions of one surface that have a slightly positive or negative polarity and are attracted or repulsed to a region on the other surface. While these may be present in a small way in tapes, they are not the main mechanism for tape adhesion.
- Diffusive adhesion – this mechanism is a merging at the molecular level of the species of one surface with the species of the other surface while still being attached to the main chain. This “entanglement” provides a level of adhesion depending on the chemical makeup of the surfaces involved. Again, not the principal way that tapes adhere.
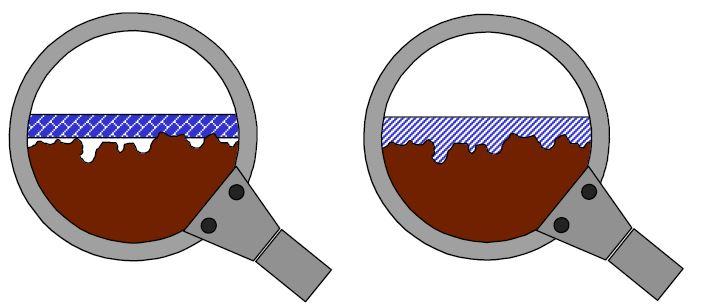
- Mechanical – mechanical adhesion or interlocking occurs when the adhesive fills the voids or the “hills and valleys” of the intended surface. This mechanism is definitely a part of what makes tapes stick. That interlocking happens primarily due to surface energy. That is the main idea we want to discuss.
Surface Energy
Surface energy is a property of a material in much the same way as density, tensile strength, or melting point. It is the attraction of the molecules of a material to its’ own molecules and to the molecules of another surface. It is an effective measure of how difficult or easy it might be to establish a bond. Surface energy determines how easy it is for the adhesive tape to “wet out” or spread over the surface and establish a bond. This intimate contact and flow over the surface and its’ topography is what allows the tape to maximize the surface area and increase adhesion.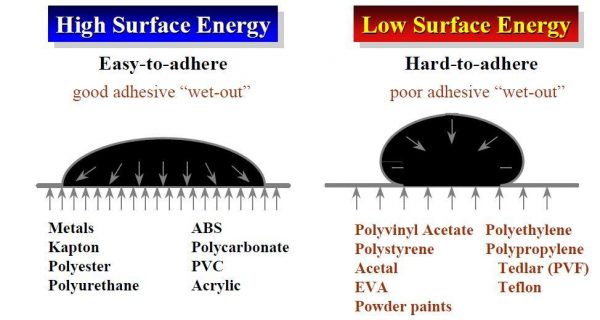
(Table courtesy of Berry Plastics)
The easiest analogy to understand surface energy is that of a waxed and unwaxed car. On a waxed car, a drop of water will “bead up” and not wet the surface. This is an example of low surface energy. Conversely, when you have an unwaxed car, a drop of water will rapidly spread out and easily establish contact. This is high surface energy. One of the reasons you see so many types of tapes even within the same family is that the adhesive chemist can deliberately modify or compound the adhesive to deal with the surface energies of the materials to be bonded. This is why the first question you will be asked about any adhesive tape application is, “What are you trying to bond to?” One of the wonderful things about the tape industry is the breadth of products available to help solve a variety of bonding challenges. Do you have questions about how to bond to a specific surface? Contact Tom Brown, Inc. to learn more.